Methodologies used by the research group
To study adaptation mechanisms and the performance of algae, we use diverse field and lab-base methods. These comprise SCUBA-diving and in-situ measurements of various physiological and abiotic parameters. Under controlled conditions in the lab, we perform ecophysiological experiments to study growth, photosynthesis and respiration. With the aid of microscopic, biochemical and molecular biological techniques, we can identify algal species and visualize their vitality, quantify stress metabolites and describe the underlying regulative processes.
Physiological Methods
Unlike metabolic processes like photosynthesis, growth integrates the influence of all positive and negative environmental conditions and thus represents the most important physiological parameter to describe performance. With the aid of in-vitro chorophyll fluorescence, growth in very low cell densities can be quickly and reliably determined. The measurement of growth curves under various abiotic environmental conditions is therefore an important step to determine and evaluate the tolerance, optima and adaptive strategies of algae.
GUSTAVS, L., SCHUMANN, R., EGGERT, A. & KARSTEN, U. (2009) In vivo growth fluorometry: accuracy and limits of microalgal growth rate measurements in ecophysiological investigations. Aquatic Microbial Ecology 55, 95-104. DOI: 10.3354/ame01291
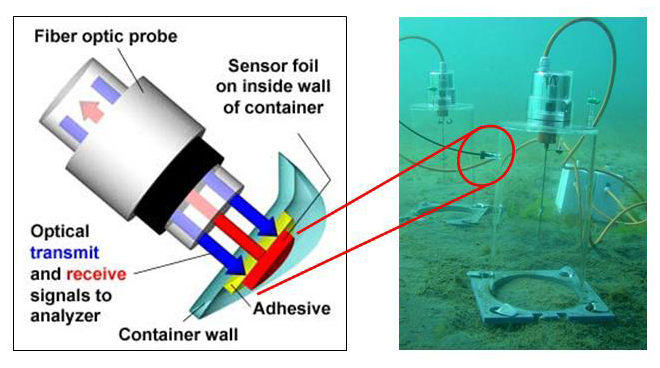
For the determination of photosynthesis and respiration as well as of primary production, we utilise an optical method. For this, the oxygen uptake in darkness and oxygen production under increasing irradiances is measured in temperature-controlled cuvettes using optodes. In the field, the primary production of benthic diatoms is registered online in a benthic chamber via optical fibres. The chambers are positioned on the sediment and anchored by SCUBA divers. One half of the chambers is irradiated by natural sunlight , which promotes the photosynthesis of the diatoms. The other half of the chambers is darkened, to follow respiration by the constant decrease of the oxygen concentration. From both data sets, the primary production rate per area and biomass parameter can be calculated. We furthermore use various fluorescence based PAM systems to determine photosynthesis parameters.
WOELFEL, J., EGGERT, A. & KARSTEN, U. (2014) Global warming could stimulate Arctic microphytobenthos primary production in Kongsfjorden (Svalbard, Norway) - in situ measurements and modelled changes. Marine Ecology Progress Series 501, 25–40.
Analytical Methods
We use various high performance liquid chromotography (HPLC) systems to separate mixtures of stress metabolites and other substances and, as far as possible, to identify and quantify them. A priority are organic osmolytes (e.g. polyols), that play an important role in salt adaptation and chemo-taxonomy. We furthermore analyse mycosporine-like amino acids (MAAs), which in many algae act as specific sunscreen substances. Our HPLC systems can also be used for the analyses of organic acids, amino acids and pigments.
KARSTEN, U., GÖRS, S., EGGERT, A. & WEST, J.A. (2007) Trehalose, digeneaside and floridoside in the Florideophyceae (Rhodophyta) – a re-evaluation of its chemotaxonomic value. Phycologia 46, 143-150.
DOI: 10.2216/06-29.1
KARSTEN, U., ESCOUBEYROU, K. & CHARLES, F. (2009) The effect of redissolution solvents and HPLC columns on the analysis of mycosporine-like amino acids (MAAs) in the macroalgal species Prasiola crispa and Porphyra umbilicalis. Helgoland Marine Research 63, 231-238. DOI: 10.1007/s10152-009-0152-0
To determine the elemental composition of our field and lab samples, we utilize a variety of equipment. For particulate components, for example, from soil crusts or for the particulate material filtrated from water samples, the Elementar Vario-EL Multi-Element analyser is used, which allows us to determine carbon and nitrogen concentrations at the microgram level simultaneously.
Analyses of carbon, phosphorous and nitrogen concentrations at the microgram level can also be performed at the Biological Station Zingst (contact: Dr. Thena Schumann): With the aid of a low temperature extraction approach - and a segmented flow analyser (Alliance Instruments) we can quantify phosphate, nitrate, nitrite and ammonium at levels from 0.05 to 10 µmol L-1. We determine the total particular and solved carbon pollution, e.g. of water bodies, with a Total Organic Carbon Analyser (Shimadzu, TOC 5000A).
BERTHOLD, M., ZIMMER, D., SCHUMANN, R. (2015) A simplified method for total phosphorus digestion with potassium persulphate at sub-boiling temperatures in different environmental samples.Rostocker Meeresbiologische Beiträge, Vol. 25, pp. 7 - 25